Why our Specific Heat Calculator
When working with physics, thermodynamics, or engineering problems, you often face the challenge of figuring out how much heat is required to warm or cool a substance. Doing these calculations by hand can be tedious, especially when juggling different units like Joules, calories, or BTUs. That’s where our Specific Heat Calculator comes in.
This article will walk you through everything you need to know about specific heat capacity: the definition, the formulas, real-world examples, a database of values, and even the most frequently asked questions. Whether you’re a student tackling physics homework, an engineer designing a heating system, or a curious cook wondering why water takes so long to boil, this guide will help you and our Specific Heat Calculator will do the math instantly.
🌡 What Is Specific Heat Capacity?
According to Britannica, specific heat capacity (often simply called specific heat) is the amount of heat required to raise the temperature of one unit mass of a substance by one degree Celsius (or one Kelvin).
The standard equation is:
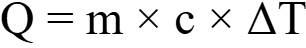
Where:
- Q = Heat energy (Joules)
- m = Mass of the substance (kg)
- c = Specific heat capacity (J/kg·K)
- ΔT = Change in temperature (°C or K)
This deceptively simple formula is at the heart of countless real-world applications. But juggling the math manually isn’t always convenient. That’s why our Specific Heat Calculator is designed to let you input known values and instantly compute the missing one.
⚙️ Why Use a Specific Heat Calculator Instead of Doing It by Hand?
At first glance, you might think: “I can just use the formula.” True but in practice, problems get tricky:
- You may need to convert units (calories to Joules, BTUs to kWh, grams to kilograms).
- Different fields use different conventions (e.g., engineers may prefer BTUs while scientists stick to Joules).
- Real-world calculations often involve large or awkward numbers.
Our Specific Heat Calculator automatically handles unit conversions, ensures precision, and saves you time. More importantly, it reduces the risk of mistakes, something especially valuable in professional or academic work.
How to Use the Specific Heat Calculator
Follow these steps:
- Enter Mass (kg)
- Enter Temperature Change (°C or K)
- Enter Specific Heat Capacity (J/kg·K)
- Choose the Output Unit
- Select Show Steps checkbox (optional)
- Click Calculate to see instant results
Tip: You can copy the result or reset the calculator to try different scenarios.
🔢 How the Specific Heat Calculator Works
The calculator has four modes. Depending on what you need, you can solve for:
- Heat Energy (Q) – How much energy is required or released.
- Mass (m) – How much material is involved given energy and ΔT.
- Temperature Change (ΔT) – How much the temperature changes for a given input of energy.
- Specific Heat Capacity (c) – The property of the substance itself.
Each time, the calculator uses the same basic equation rearranged:
Q = m × c × ΔT
c = Q / (m × ΔT)
m = Q / (c × ΔT)
ΔT = Q / (m × c)
👉 Instead of working this out manually, you just enter what you know into the calculator, pick your units, and get an instant, accurate result.
🧮 Worked Examples with the Specific Heat Calculator
Let’s walk through some real-world examples using our calculator.
Example 1: Heating Water
You want to heat 2 kilograms of water by 30 °C. Water’s specific heat is about 4186 J/kg·K.
Formula:
Q = m × c × ΔT = 2 × 4186 × 30 = 251,160 J (≈ 251 kJ)
Using the Specific Heat Calculator, you’d enter:
- Mass = 2 kg
- c = 4186 J/kg·K
- ΔT = 30 °C
- Output in Joules
Result: 251,160 J.
Example 2: Cooling Aluminum
A block of 0.5 kg aluminum cools by 50 °C. Aluminum’s specific heat is ~897 J/kg·K.
Q = 0.5 × 897 × 50 = 22,425 J
So it requires about 22 kJ of energy loss to cool it.
Example 3: HVAC Application
You want to heat 100 m³ of air (about 120 kg) by 10 °C. Air’s specific heat ≈ 1005 J/kg·K.
Q = 120 × 1005 × 10 = 1,206,000 J (≈ 1.2 MJ)
That’s a lot of energy, which is why heating/cooling systems require careful design. A Specific Heat Calculator saves time here, especially with large datasets.
Example 4: Kitchen Science
You’re boiling 1 liter of water (≈ 1 kg) from room temperature (25 °C) to boiling (100 °C).
Q = 1 × 4186 × (100 – 25) = 313,950 J (≈ 314 kJ)
No wonder pasta takes a while the specific heat of water is very high!
📊 Common Specific Heat Values
Different substances have vastly different specific heats. Here are a few important ones (from Engineering Toolbox):
| Substance | Specific Heat (J/kg·K) | Notes |
|---|---|---|
| Water | 4186 | High capacity, stabilizes climate |
| Aluminum | 897 | Common in engineering |
| Copper | 385 | Excellent heat conductor |
| Iron | 449 | Found in many industrial uses |
| Air (at 25 °C) | 1005 | Important for HVAC design |
| Wood | 1300 – 2400 | Varies by type, used in building and fuel calculations |
| Steel | 490 | Common structural material, retains heat moderately |
| Potassium | 1000 | Used in chemical and laboratory applications |
| Ice | 2100 | Lower than liquid water |
Tip: Our Specific Heat Calculator allows you to input these directly and switch units instantly.
🌍 Applications of Specific Heat in Real Life
- Climate Science – Oceans absorb heat thanks to water’s high specific heat, stabilizing Earth’s climate (NASA Climate). Engineering, Car engines, HVAC systems, and metallurgy all rely on precise heat calculations. For phase change energy, you can explore Latent Heat Calculator by Omni Calculator to see how much energy is needed during melting or freezing.
- Engineering – Car engines, HVAC systems, and metallurgy all rely on precise heat calculations.
- Medicine – Cooling packs and thermal therapy use materials with specific heat to regulate temperature.
- Cooking – Explains why oil heats faster than water and why cast iron pans retain heat.
Each of these industries can save hours of manual calculation using our Specific Heat Calculator.
❓ Frequently Asked Questions About Specific Heat Calculator
What is a Specific Heat Calculator?
It’s an online tool that quickly calculates heat energy, mass, temperature change, or specific heat capacity using the formula Q = m × c × ΔT.
Why does water have a high specific heat?
Because of hydrogen bonding, water requires a lot of energy to raise its temperature. This is why oceans regulate climate.
How do I calculate specific heat manually?
Use c = Q / (m × ΔT). For example, if 2000 J of heat raises 1 kg of a material by 2 °C, then c = 2000 / (1 × 2) = 1000 J/kg·K
Can specific heat be negative?
No. Specific heat capacity is always positive, it measures how much heat is absorbed per degree of temperature increase.
Which material has the highest specific heat?
Water is among the highest (4186 J/kg·K). That’s why it plays a key role in thermal regulation.
Can this calculator work for calories, BTUs, or kWh?
Yes! The Specific Heat Calculator supports multiple units including Joules, calories, kilocalories, BTUs, watt-hours, and more.
How accurate is the Specific Heat Calculator?
It uses precise conversion factors and SI standards (see NIST). The only limit is the accuracy of the values you input.
🏁 Conclusion: Why You Should Use This Calculator
The Specific Heat Calculator is more than just a convenience, it’s a necessity for students, teachers, scientists, and engineers who regularly deal with heat energy problems. It saves time, reduces errors, and ensures accurate results across multiple unit systems.
By combining an easy-to-use interface with a rich knowledge base (like the article you’re reading now), it’s not just a calculator, it’s a full learning tool.
So whether you’re boiling water, designing an HVAC system, or studying thermodynamics, remember: you don’t need to juggle numbers manually. Our Specific Heat Calculator is here to do the heavy lifting.
