Introduction
When you have spent over two decades working with wood stoves, you get used to answering all sorts of questions. I have been asked everything from how to split a stubborn piece of oak to how to keep a fire going overnight in the dead of winter. But one question keeps coming up, especially from homeowners who are curious about the science behind their heat source. They ask, sometimes with a half-smile like they are back in school again, is burning wood a chemical change?
I always smile when I hear it, because it is one of those questions with a short answer and a long story. The short answer is yes, burning wood is a chemical change. But the longer answer is where the magic lies, because understanding why it is a chemical change opens up a whole world of insight into how your stove works, how to get the most heat out of every log, and even how to keep your home safer and cleaner.
So, grab a seat by the stove, and let’s talk about what really happens when you light that fire.
What Happens During Wood Combustion
I have lit thousands of fires in my career, from testing a newly installed stove in a mountain cabin to coaxing a reluctant fire into life during a home efficiency demo. No matter how many times I watch it, the process still fascinates me.
When you strike a match and set kindling ablaze, you are starting a process called wood combustion chemistry. It is not just burning in the everyday sense, it is a complex sequence of chemical reactions that transforms solid wood into heat, light, gases, and ash. . Understanding is burning wood a chemical change helps you appreciate every stage of this process.
Let’s break it down in plain terms:
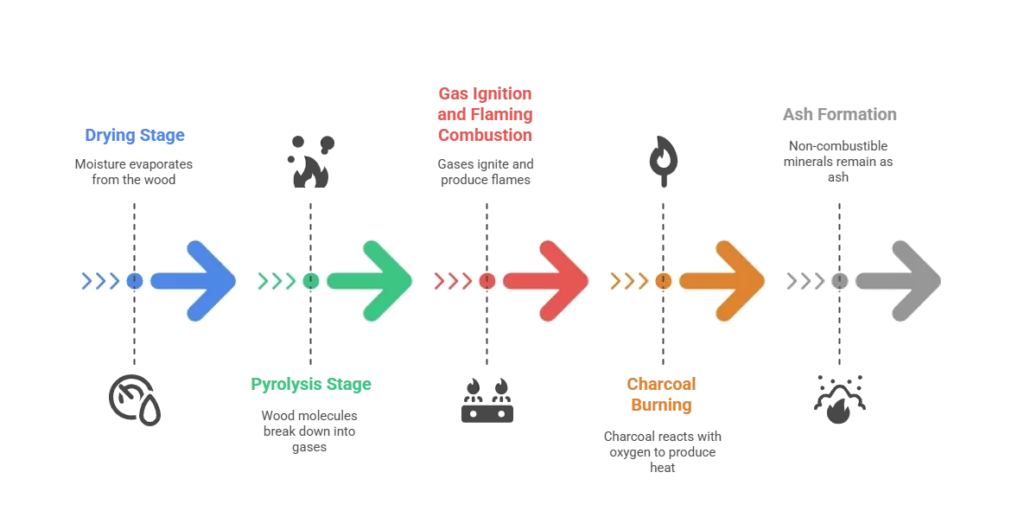
- Drying Stage
Even seasoned firewood has some moisture inside. As the heat rises, the water turns into steam and escapes through the wood fibers. This is a physical change, because the water molecules are the same before and after. If your wood is too wet, you will waste a lot of energy just getting rid of that moisture, and your fire will struggle to get going. Even here, thinking about is burning wood a chemical change reminds you that this is not yet the main chemical reaction. - Pyrolysis Stage
Once the wood’s temperature hits around 300 to 500 degrees Fahrenheit, the heat starts to break apart the cellulose and lignin molecules in the wood. This produces flammable gases like methane, hydrogen, and carbon monoxide. Here is where is burning wood a chemical change comes into play, the structure of those original molecules is being permanently altered. For more detailed research on how wood components break down under heat, you can refer to the USDA Forest Service study, which explains the chemical reactions involved in wood pyrolysis. - Gas Ignition and Flaming Combustion
The gases released during pyrolysis ignite and create those dancing flames we love to watch. Carbon atoms bond with oxygen to form carbon dioxide, hydrogen bonds with oxygen to form water vapor, and many other chemical reactions happen at the same time. These changes cannot be reversed. - Charcoal Burning
When the flames die down, you are left with glowing charcoal, mostly pure carbon. This carbon slowly reacts with oxygen, producing steady heat and a red-orange glow. - Ash Formation
What remains after all this is ash, made up of non-combustible minerals like calcium, potassium, and magnesium. It is the quiet reminder of the fire’s work, and you cannot turn it back into wood no matter what you do.
The Science Behind Wood Burning Chemistry
Wood is a mix of organic polymers, mainly cellulose, hemicellulose, and lignin along with small amounts of resins, tannins, and minerals. When people ask me what happens when wood burns chemically, I tell them to imagine those polymers as long chains of building blocks. When heat is applied, those chains snap apart, and the building blocks rearrange themselves into entirely new structures. This is why the question of is burning wood a chemical change is such a fundamental concept.
The main chemical reaction for burning cellulose, simplified, is:
But real wood burning is far more complicated. In the real world, you have hundreds of different compounds reacting at the same time, each at its own temperature and rate. Some reactions produce those beautiful yellow flames, others produce smoke and soot if the burn is incomplete.
Physical vs Chemical Changes in Wood
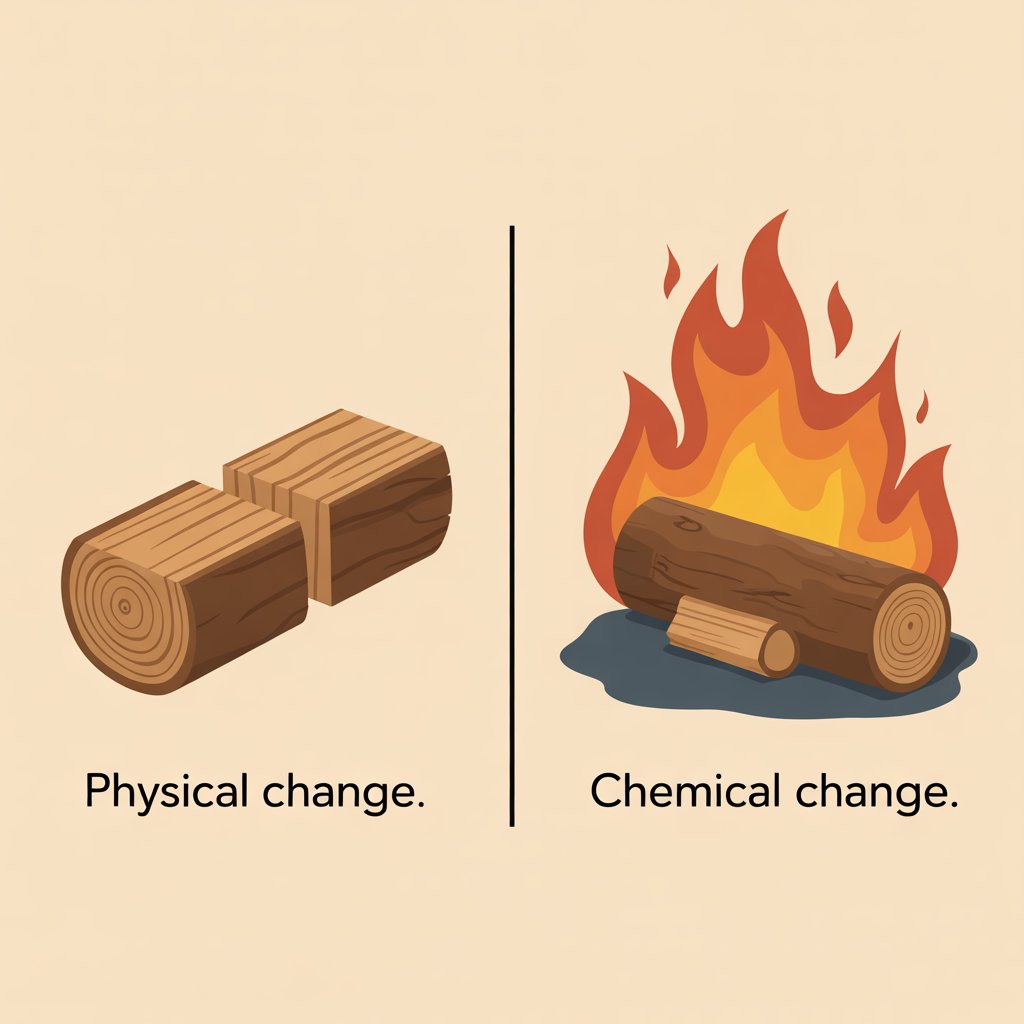
A lot of homeowners confuse these two ideas. I always explain it like this:
Physical changes affect how something looks or feels, but do not change what it actually is. Chemical changes create new substances that were not there before.
- Physical change examples: Splitting a log into smaller pieces, stacking it in your shed, or drying it so the moisture content drops. You still have the same wood chemically.
- Chemical change examples: Burning that wood in your stove, letting it rot in a damp pile, or charring it into biochar. The chemical structure changes and new materials form.
Think about baking bread. You can slice it, freeze it, or toast it. Those are physical changes. But when you actually bake dough, the yeast produces gases, the proteins change, and you get bread. There is no going back to raw dough, just like there is no going back to unburned wood once it has turned to ash. . This comparison helps people grasp why is burning wood a chemical change.
Temperature Stages of Combustion
Over the years, I have learned to read a fire’s temperature almost like a musician hears notes. If you want efficient heat, you have to know the stages.
- Below 212°F (100°C): Moisture is leaving the wood, physical change only.
- 300–500°F (150–260°C): Pyrolysis kicks in, chemical changes start.
- 500–1,100°F (260–593°C): Volatile gases ignite, flames dance, chemical changes dominate.
- 1,100–1,800°F (593–982°C): Complete combustion possible, maximum efficiency.
- Above 1,800°F (982°C): Long exposure can damage certain stove parts.
Maintaining the right heat is crucial. Too cool and you waste energy, too hot and you risk your stove’s health.
Wood Species and Chemical Composition
Not all wood burns the same. Hardwoods like oak and maple have more lignin and produce longer-lasting coals. Softwoods like pine ignite quickly and give you that big burst of flame early in the burn.
In my own home, I often mix species. A bit of pine or spruce to get the fire going, followed by solid hardwood splits to carry the heat through the evening. Once you understand wood burning chemical reaction principles, you can start treating your firewood pile like a chef’s spice rack, each log has a role to play.
Complete vs Incomplete Combustion
The difference between complete and incomplete combustion is one of the most important lessons I teach stove owners.
- Complete combustion: All the carbon and hydrogen in the wood are fully oxidized into CO₂ and H₂O. You get more heat, less smoke, and a cleaner chimney.
- Incomplete combustion: Caused by low temperatures, poor airflow, or damp wood. It wastes fuel, produces more smoke, and creates creosote that can cause chimney fires.
A good rule of thumb is to check your chimney. If you see thick, white or gray smoke, your fire is not burning efficiently. The ideal is little more than a faint shimmer of heat waves.
Environmental Aspects
When people ask me if wood combustion is reversible, I have to be honest. no, it is not. Once you have turned wood into heat, gases, and ash, there is no going back. But that does not mean wood burning is bad for the planet.
Burning sustainably harvested, well-seasoned wood in an efficient stove can be carbon neutral, because the CO₂ released is roughly equal to what the tree absorbed while growing. The real problems come from incomplete combustion, which produces particulates and volatile organic compounds.
This is why modern EPA-certified stoves are built to burn hotter and cleaner. They keep combustion gases in the burn zone longer, which means more chemical changes happen completely and fewer pollutants escape.
For more information, see EPA Wood Smoke and Your Health
Practical Applications for Wood Stove Users
Knowing that burning wood is a chemical change is not just trivia, it can make you a better fire keeper.
Here are my top five tips:
- Use seasoned wood with a moisture content under 20 percent.
- Maintain proper airflow so the fire has enough oxygen for complete combustion.
- Burn hot fires rather than letting logs smolder.
- Mix species for quick ignition and steady heat.
- Clean your chimney regularly to remove creosote and prevent fires.
If you want more details, you can learn more about proper wood seasoning techniques or check our guide on wood stove maintenance tips.
FAQs About Is Burning Wood a Chemical Change
Is burning wood a chemical change or a physical change?
It is a chemical change, because it alters the wood’s molecular structure and creates new substances like carbon dioxide, water vapor, and ash.
What happens when wood burns chemically?
Cellulose, hemicellulose, and lignin break down into gases that ignite, producing heat, light, and new compounds that did not exist in the original wood.
Can you reverse the wood burning process?
No, once the chemical bonds have been broken and rearranged into new substances, you cannot recreate the original wood.
Does wood stove efficiency depend on combustion chemistry?
Absolutely. Understanding wood combustion chemistry helps you burn cleaner, hotter, and more efficiently.








